- Review
- Open access
- Published:
Management of multiple and unruptured cerebral aneurysms
Egyptian Journal of Neurosurgery volume 37, Article number: 26 (2022)
Abstract
The incidence of multiple aneurysms was 10.7–34% of CA. Multiple associated factors were found; hypertension was the most significant one and others like advancing age and female sex were also documented. The estimated prevalence of UA is 5–10%. They include those aneurysms that did not rupture and discovered incidentally and those presented with symptoms rather than SAH, e.g., cranial nerve palsy or mass effect. Unruptured intracranial aneurysms are diagnosed with higher frequency nowadays as a result of imaging techniques improvement. The reported annual rate of rupture of UA is approximately 0.7–1%. The natural history of unruptured cerebral aneurysms cannot be extrapolated from the evaluation of individuals with ruptured aneurysms. Multiple cerebral aneurysms pose an even greater risk than a single aneurysm; the risk of rebleeding from the original aneurysm is larger and occurs sooner. The natural course of the disease has led to a consensus that all multiple unruptured aneurysms should be treated when technically viable. However, the prophylactic treatment of multiple unruptured is still controversial. Weighing the risk of intervention to the risk of observation is a mandatory pathway. Factors like age of patients, size and location of the aneurysms influence the decision-making and the type of therapy to be elected.
Background
Cerebral aneurysms
Cerebral aneurysms (CA) are outpouching of the parent artery. They mostly originate at the arterial bifurcation. They do occur in 1–2% of the population and represent about 80% of non-traumatic subarachnoid hemorrhages (SAH) when they rupture. Autopsies showed their prevalence in the adults between 1 and 5%, though 50–80% of all aneurysms remain unruptured during a person’s lifetime. However, CA are sporadically acquired lesions; the genetic predisposition of CA and familial occurrence is documented [1,2,3,4].
Multiple aneurysms
The incidence of multiple aneurysms was 10.7–34% of CA. Multiple associated factors were found; hypertension was the most significant one and others like advancing age and female sex were also documented. Multiplicity was also associated with the familial occurrence of CA and the autosomal dominant polycystic kidney disease [4, 5].
Unruptured aneurysms (UA)
Epidemiology
The estimated prevalence of UA is 5–10%. They include those aneurysms that did not rupture and discovered incidentally and those presented with symptoms rather than SAH, e.g., cranial nerve palsy or mass effect. Unruptured intracranial aneurysms are diagnosed with higher frequency nowadays as a result of imaging techniques improvement. Unruptured intracranial aneurysms are more common in female with a 3:1 ratio to male [6, 7].
Pathophysiology
The genetic predisposition for CA is documented in many studies; one of these famous studies is the familial intracranial aneurysm (FIA) study. Multiple genes were presumed to have strong association, as the collagen genes (type 1A2, III), CDKN2B antisense inhibitor gene, the SOX17 transcription regulator gene, and the EDNRA gene. Autosomal dominant polycystic kidney disease, Marfan syndrome, fibromuscular dysplasia, Ehlers–Danlos syndrome, sickle cell disease, and arteriovenous malformations are contributing conditions too. The most common histological defects are in the tunica media of the arterial wall, which, in combination with hemodynamic factors, lead to aneurysmal dilatation at arteries branching sites. Precipitating factors like smoking, alcohol abuse, and hypertension may increase the risk for rupture and subsequent SAH [4, 8].
Clinical presentation
Unruptured intracranial aneurysms might be discovered by chance as a result of unrelated complaints, or they can be noticed as they expand and pressure nearby brain structures. Aneurysms in the middle cerebral artery can cause hemiparesis, visual field defects, or seizures; posterior communicating artery or basilar artery aneurysms can cause third cranial nerve palsy; cavernous sinus aneurysms can cause cavernous sinus syndrome; basilar distribution aneurysms can cause brainstem compression; and, on rare occasions, an embolus from the aneurysmal sac can cause transient ischemia or permanent stroke [9, 10].
Diagnosis
CT angiography (CTA), magnetic resonance angiography (MRA), and catheter angiography, which is regarded the gold standard, are the radiographic tests available to characterize the size and morphologic aspects of an intracranial aneurysm [11].
CTA shows brain arteries in three-dimensional views using thin-section contrast-enhanced CT and software-generated images. It is a noninvasive procedure. These reconstructed images take only a few minutes to create and enable for the evaluation of the vasculature in close proximity to the brain and the bones of the skull base, making surgical planning easier. CTA has a sensitivity range of 95% and specificity 83%. It can detect aneurysm as small as 2.2 mm. Because a large bolus of contrast material is frequently delivered, CTA has a limited utility in patients with poor renal function [11,12,13].
For the detection of cerebral aneurysms, MRA is extremely sensitive and specific. The capacity to detect aneurysms and measure the size, rate, and direction of flow in an aneurysm relative to the magnetic field is provided by magnetic resonance arteriography (MRA). It also aids in the visualization of thrombosis and calcium deposits within an aneurysm. It has a sensitivity of roughly 86% for aneurysms larger than 3 mm and a false-positive rate of about 16%. Because the false-positive rate is substantial, this method may be beneficial for screening high-risk patients, such as aneurysm sufferers' first-degree relatives. Within the first 24–48 h, magnetic resonance imaging (MRI) alone is not sensitive. MRI is useful for diagnosing sub-acute to distant SAH after 4–7 days. In the case of several aneurysms, it may be useful to figure out which one bled [11, 14].
Catheter angiography or digital subtraction angiography (DSA), with or without sophisticated three-dimensional capability, is the gold standard for evaluating an aneurysm's relationship to adjacent vessels in detail. Catheter angiography, on the other hand, is more expensive and invasive than either MRA or CTA. Even in the hands of expert operators, it carries the danger of neurologic problems, which occur in 1.0–2.5% of cases and result in permanent disability in 0.1–0.5%. Other concerns include femoral artery injury (0.05–0.55%), groin hematoma (6.9–10.7%), and contrast-induced renal consequences (1–2%) [15].
Because of their noninvasive nature, brain MRA or CTA are the procedures of choice for screening unruptured aneurysms; nonetheless, a cerebral angiography is often utilized to further explain the features of an aneurysm. It is worth noting that MRA and CTA's ability to provide such morphological information is quickly improving. MRA is the screening method of choice for those individuals for whom contrast administration is contraindicated (including patients with renal insufficiency for whom MRI-related gadolinium contrast is prohibited) since gadolinium contrast administration is not required [14, 16].
Management of cerebral aneurysm
Treatment for cerebral aneurysms has progressed over time. In 1885, Victor Horsely was the first to perform a Hunterian ligation on a huge ICA aneurysm. Harvey Cushing invented muscle wrapping and suture ligation for brain aneurysms a few years later. Walter Dandy performed the first aneurysm cutting with a silver clip in 1937. Surgical clipping of cerebral aneurysms has advanced significantly during the last century, thanks to advancements in surgical equipment, microscopes, intraoperative monitoring, and clip design [17,18,19].
Endovascular therapy of cerebral aneurysms has progressed in tandem in the second part of the twentieth century. Surgical clipping has been replaced by coil embolization as a therapy option. The technique's feasibility, as well as the variety of coil sizes and types that have emerged, has fueled this trend in the years since 2003. Endovascular embolization of cerebral aneurysms has progressed from the use of detachable balloons and onyx 500 in the past to coils now, with the Woven EndoBridge (WEB) devices being the most current advances. Flow diverters are a significant advancement in the treatment of big and wide neck aneurysms using endovascular techniques [20, 21].
Clipping versus coiling
Many factors influence the therapy of cerebral aneurysms, including (1) the patient’s neurological condition, (2) aneurysm-related characteristics (size, anatomy, location, multiplicity, status), and (3) the therapist's ability (interventionist or surgeon). Dome/neck ratios less than 1 and large neck aneurysms (> 3–4 mm) made coil embolization problematic. The basilar apex is a good location for coiling aneurysms, but MCA aneurysms may be difficult to coil due to a branch near the neck. Associated intracranial hemorrhage: surgery enables for both hemorrhage evacuation and aneurysm repair. Clipping, rather than coiling, improved the outcome of third cranial nerve palsy, according to Levio et al. and Feely et al. [22, 23]. Patients who are over the age of 65 or who are on Plavix or other anticoagulants may benefit from coiling.
Management of multiple and unruptured cerebral aneurysm (MUA)
Natural history
The natural history findings from the international study of unruptured intracranial aneurysm (ISUIA) and other studies support the assumption that the natural history of unruptured cerebral aneurysms cannot be extrapolated from the evaluation of individuals with ruptured aneurysms. The patients included in the ISUIA study phase II were as follows: UA in patients without SAH from another aneurysm (group 1) and patients with SAH from another aneurysm (group 2) [7, 24].
Multiple cerebral aneurysms pose an even greater risk than a single aneurysm because patients with multiple lesions have a higher related death rate. Furthermore, in this group of patients, the risk of rebleeding from the original aneurysm is larger and occurs sooner. MUA patients are more likely to develop de novo CA and have their existing CA increase in size further. The natural course of the disease has led to a consensus that all aneurysms should be treated when technically viable and patient should be followed up for a considerable duration [25,26,27].
The risk of aneurysmal rupture without any intervention should be weighed against the hazards of surgical clipping or endovascular treatment in the case of an unruptured intracranial aneurysm. (1) Aneurysmal factors such as location, size, morphology, whether a thrombus exists within the aneurysm, and the presence of a daughter sac or multiple lobes, and (2) patient factors such as age, medical history, history of subarachnoid hemorrhage, and family history of subarachnoid hemorrhage should be considered [28].
Other factors that can affect rupture risk include aneurysm location and patient characteristics such as elder age and hypertension [7]. CA location on the anterior or posterior communicating artery, as well as the presence of a daughter sac, was also a risk factor for rupture [29]. Age over 60 years, female sex, Finnish or Japanese ancestry, aneurysm size over 5 mm, posterior circulation location, and symptomatic unruptured aneurysms all had a higher risk of rupture, according to a major meta-analysis of currently available research [30].
The previously described risk factors for aneurysmal rupture have a significant impact on whether or not to treat an UA. UA that are about to burst can be clipped if they display progressive cranial nerve paralysis, a rise in size on serial CTA or DSA, or the beating aneurysm sign (pulsatile change in aneurysm size between imaging cuts of CTA or CTA). Endovascular treatment for aneurysms that have not ruptured is still controversial. Pipeline flow diverters, on the other hand, are now the widely used treatment for giant UA.
The reported annual rate of rupture of UA is approximately 0.7–1% [31, 32]. According to ISUIA, a patient without a history of SAH, the annual rate of rupture of an aneurysm less than 10 mm in diameter was 0.05%, whereas it was 0.5% in a patient with a history of SAH [7]. The natural history of individuals with aneurysms 3 mm or larger and an annual rupture rate of 0.95% was described in a Japanese prospective study [29]. However, Schievink et al. and Orz et al. [33, 34] found that the majority of aneurysms smaller than 6 mm in diameter were ruptured, and they concluded that an unruptured tiny aneurysm is not granted to be harmless and should be operated on in carefully selected patients. Juvela et al. [34] found a 1.4% annual rupture rate for previously UA, with cumulative bleeding rates of 10, 26, and 32% in 10 years, 20 years, and 30 years after diagnosis. Yasui et al. [35] observed that 14.5% of patients who received follow-up evaluation and were treated conservatively experienced bleeding from previously UA and the deaths of 53% of those patients. They also discovered that the overall annual rupture rate was 2.3%, with cumulative rates of bleeding being 20% for all patients 10 years after diagnosis and 35% at 15 years. They came to the conclusion that an unruptured aneurysm had a high risk of rupture [35]. Mizoi et al. [36] discovered that eight patients (16%) had an intracranial bleeding event with absolute or probable proof of aneurysm rupture in 49 patients with unruptured aneurysms managed conservatively. The bleeding was fatal in seven of the eight individuals (87.5%).
The familial history of CA is sparse. In a regression study (ISUIA), it is found that a family history was not predictive of bleeding. However, according to the FIA study, 548 first-degree unaffected relatives of patients with a familial history of CA had MRA screening: during follow-up, two patients suffered aneurysmal rupture, resulting in an annual rupture rate of 1.2 per 100 patients (95% CI 0.1–4.3) [4, 7]. After matching for aneurysm size and location, the rupture rate in Ajiboye et al. [24] was seventeen times greater than in ISUIA. The little, however, due to the low number of ruptures and wide 95% confidence intervals, conclusive conclusions about rupture rates in familial aneurysm could not be drawn [37]. Aneurysms that present with SAH bleed again at a rate of 9% within the first 3 days [38]. Because of the increased risk of bleeding of 6% per year [39], patients with known CA who diagnosed with brainstem dysfunction or cranial nerve palsies should be examined and managed as soon as possible.
ISUIA
Unruptured cerebral aneurysms have a different risk of bleeding than ruptured aneurysms. The following elements influence the natural history and treatment outcomes: 1 thru 4—Factors affecting patients Smoking 6 Associated medical conditions Associated age of the patient Previous subarachnoid hemorrhage history (SAH)—Characteristics of aneurysms Dimensions. The most important predictor for future rupture 2 is the predicted annual risk of rupture, which is 0.05% for aneurysms less than 10 mm in diameter and 1% or higher for aneurysms larger than 10 mm in diameter, according to the original ISUIA study results from 1998. 2 An ISUIA follow-up research from 2003 7 found that patients with unruptured aneurysms had 5-year cumulative rates in the internal carotid artery (ICA), ACOM, anterior cerebral artery (ACA), and middle cerebral artery (MCA) For aneurysms less than 7 mm, 7–12 mm, 13–24 mm, and 25 mm or greater, the rates are 0%, 2.6%, 14.5%, and 40%, respectively, compared to 2.5%, 14.5%, 18.4%, and 50%, respectively, for the same size categories involving posterior circulation and posterior communicating artery (PCOM) aneurysms. The diameter of the relative risk is 1.11/mm. 6 Address Unruptured aneurysms with PCOM, vertebrobasilar, and basilar terminations are more prone to rupture. Aneurysm morphology: Aneurysms that are irregular or multilobular are more prone to burst (Table 1) [7].
Therapeutic options
Surgery
According to ISIUA: 1917 patients with mean age 50 years with 32.3% of them being harboring multiple aneurysm underwent surgery. At 1 year, total morbidity and death rates in patients with open surgical repair were 10.6–12.6%. The age of the patient is a significant influence in overall surgical result, with a significant increase in risk for those 50 years and older, and a significant increase in risk after the age of 60–70 years. Large aneurysmal size, position in the posterior circulation (especially basilar tip), history of ischemic cerebrovascular illness, and presence of aneurysmal symptoms other than rupture are further indicators of poor surgical prognosis (Table 2) [7].
The goal of surgery is to exclude the aneurysmal pouch from the circulation and parent artery wall. Surgical clip occlusion of the neck is the main stranded to achieve this. Other surgical maneuvers could be used as wrapping the aneurysm, trapping the parent artery, or surgical bypass could be helpful in cases of tiny blood blister-like aneurysm or giant aneurysms incorporating the parent artery or other arterial branches. Orz et al. [33] reported the surgical treatment of unruptured aneurysms in 310 patients, of whom 57 had multiple aneurysms as follows: aneurysms were wrapped in 21 patients, and parent artery trapped in 7, and trapped after bypass surgery in 3, and the rest were clipped. No neurological deficits remained in 262 patients (84.5%), and “death,” in one patient (0.3%). The favorable outcome was documented with the following: 1—patient 60 years old or younger, 2—small- (< 6 mm) or medium-sized (6–15 mm) aneurysms, 3—anterior circulation aneurysms, 4—single UA (96% while 88% in MUA). Drake11 discovered a 14.3% morbidity rate in the posterior circulation after surgery for MUA unruptured asymptomatic aneurysms, compared to 0% morbidity in the anterior circulation [39].
From previous studies, the surgical morbidity was significantly higher in cases of multiple aneurysm; this can be justified by further brain manipulation either in one or more sitting. Giant size (> 15 mm) and elder patients (60–70 years old) carry higher risk of surgical morbidity and subsequently are poor surgical candidates. Presence of CA in locations not amenable and technically challenging for surgical access as cavernous carotid, vertebrobasilar trunk or basilar apex magnifies the risks too. On the other hand, MCA is favorable and accessible location for surgical clipping. Finally, surgical experiences could explain the variability in the outcomes of these studies [40].
Endovascular
-
1.
Coil embolization: Packing the aneurysmal sac with different types of coils. For more challenging cases and wide neck aneurysms, coiling the aneurysm with or without assisting procedures such as balloon inflation or stent implantation at the aneurysm neck can be sought. The intraluminal conventional stents that aid in coiling are categorized as follows: (1) open-cell design (high elasticity), such as the Neuroform stent, and (2) closed-cell design, such as the Enterprise stent. The LVIS stent is a modified closed-cell design stent that serves as a bridge between regular stents and flow diverters by providing higher metal coverage (23%) [41, 42].
Xun Shen et al. [43] showed 36 individuals with multiple aneurysms (84 aneurysms in total), 82.2% of which were unruptured. Fifty-four (85.7%) of the 63 aneurysms treated using coil embolization were smaller than 15 mm in diameter, seven (11.1%) were 15–25 mm, and two (3.2%) were greater than 25 mm. Fifteen aneurysms (23.8%) had necks of 4 mm or smaller, 46 aneurysms (73.0%) had wide necks, and two aneurysms (3.2%) were fusiform. Six ICA aneurysms, five MCA aneurysms, two PCoA aneurysms, two ACoA aneurysms, and two anterior cerebral artery aneurysms were treated with stent-assisted coiling; one VA-PICA aneurysm was stented alone; and four lesions (three gigantic ICA aneurysms and one VA-PICA aneurysm) were treated with parent artery occlusion. Total occlusion was achieved in 54 (85.7%) [43]. In the ISUIA research, 451 patients treated with endovascular coiling had a 1-year morbidity rate of 6.4% and a death rate of 3.1%. The endovascular group had different baseline characteristics than the surgery group (which comprised older patients, larger aneurysms, and a higher number of posterior circulation aneurysms); therefore, the findings are not directly comparable (Table 3) [7, 44].
Table 3 Risks and results of endo-vascular coiling in cases of unruptured aneurysms [52] An endovascular method may be unfeasible due to prohibitive tortuosity in the access vessels. In rare circumstances, combining an open surgical approach with a close proximity approach to the lesion may be appropriate. The presence of a daughter dome in the aneurysm neck may make an endovascular cure more difficult to perform. The parent artery is included. Because of the partial thrombosis of the aneurysm, recurrence of the lesion is highly possible due to the shifting and compaction of the coil mass. The significance of endovascular therapy in the treatment of aneurysms that have a mass effect is debatable. Intuitively, only an open surgical technique appears to have a possibility of alleviating mass effect symptoms. However, there is some indication that cranial neuropathies improve the following endovascular coil occlusion (Fig. 1) [40, 45].
Fig. 1 Illustration for coil embolization in a rubber model [44]
-
2.
Onyx HD 500; ev3 Neurovascular: is an embolic agent used to treat giant and wide-necked aneurysms that cannot be treated with surgical clipping or endovascular coiling. However, due to poor outcomes recorded in several trials such as the Cerebral Aneurysm Multicenter European Onyx (CAMEO), its use has been recently declined [46].
-
3.
Flow diverters: With the new endovascular concept focusing on reconstructing the parent vessel wall rather than embolization of the aneurysmal sac, diverters are innovative stents with higher pore density (smaller pores than ordinary stents) (0.02–0.05 mm2) and more elastic to fit the vessel profile (higher metal coverage), resulting in flow stagnation and gradual thrombosis inside the aneurysm. After stent-assisted coiling and flow diverter embolization, dual antiplatelet is administered as a post-procedural need. Although pipeline embolization is commonly used to treat many big ICA aneurysms, there have been reports of spontaneous aneurysm rupture and intraparenchymal hemorrhage following the procedure (0.6% and 2.4%, respectively) [20, 47].
-
4.
Web devices: They are inflatable intra-saccular flow disrupters with a unique design. A regular microcatheter is used to deliver the drugs to the aneurysm, and radiopaque markings on the catheter's surface aid in their appropriate placement. They are made up of a barrel and spherical nitinol braid on the inside and an outer nitinol braid on the outside (Fig. 2) [48, 49].
Fig. 2 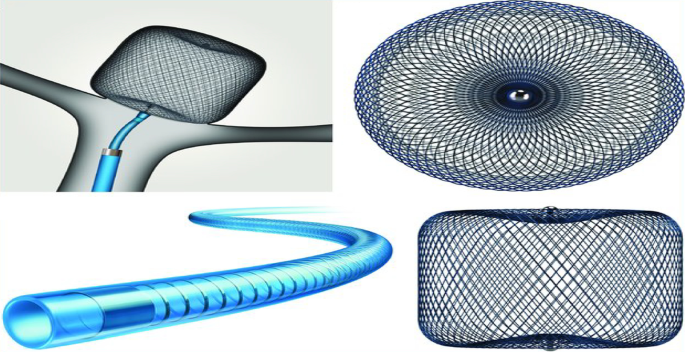
(This figure was uploaded by Adam A Dmytriw Content may be subject to copyright) [48]
Illustration for Woven EndoBridge (WEB; Microvention) device
Multimodal approach
When open surgery and endovascular methods are combined in same or different sitting, they may be beneficial to one other in the treatment of MUA. To maximize the benefit to the patient, researchers are attempting to strike a delicate balance between enhancing aneurysm obliteration and decreasing treatment-related morbidity. This could comprise parent artery reconstruction or bypass treatments followed by coil implantation, or vice versa. Creating a surgical access to coil embolization through a trans-cranial approach was reported too [40, 50].
Conservative treatment
Conservative approach could be sought in newly diagnosed MUA, especially those patients over the age of 60 years and for small (< 7 mm) aneurysms, except in those with a strong family history of aneurysmal rupture [50]. It also can be individualized according to patient condition and wish after informative counseling and restrict follow-up regimen. Routine periodic imaging with MRA or CTA on annually for about 3 years at least should be done. Patient with documented aneurysmal growth radiological or clinical (development of new symptoms) should be considered for intervention. Alleviating other risk factors is a cornerstone: control of hypertension, moderate alcohol consumption, and avoidance of cocaine abuse [51].
There is no indication that anticoagulant therapy increases the risk of SAH in patients with aneurysms. Anticoagulant medicines should be avoided in patients who have an unruptured aneurysm because they increase the chance of a poor result if the aneurysm ruptures. Many studies have found that individuals using aspirin have a lower risk of rupture. However, it is uncertain whether the benefit of aspirin therapy in individuals with an unruptured intracranial aneurysm justifies the hazards, and a clinical trial is needed to clarify. In a case–control study of cases of intracerebral hemorrhage and subarachnoid hemorrhage comparing subjects who use aspirin to those who not, patients who took aspirin had the lowest risk of aneurysm rupture during follow-up in an examination of data from the ISUIA with regard to the untreated patient [52, 53].
Our own experience
Case 1
A male patient, 56 years old, presented to our outpatient clinic. He complained of chronic mild headache, 3rd nerve palsy in the form of partial ptosis. MRI and MRA revealed a prebifurcational carotid aneurysm; it was detailed later on DSA. After FU for 6 months, growth by 2 mm was confirmed. Endovascular coil embolization was elected for the patient. The patient was discharged neurologically intact and with significant improvement on follow-up visits (Fig. 3).
Case 2
A female patient, 52 years old, presented with recurrent episodes of headache to our outpatient clinical. CT brain revealed filling defect in the right Sylvain fissure suspicious of CA. CTA was done and revealed right bifurcation MCA aneurysm with the widest diameter being more than 1.3 cm, and another left M1 small aneurysm less than 4 mm in the widest dimension. Surgical clipping was done for the right-sided aneurysm. Patient was discharged on regular follow-up scheduled radiology for the other left MCA aneurysm. Two years later, CTA revealed significant growth of the left M1 aneurysm and surgical clipping was decided for the patient. The patient was discharged neurologically intact and a new angiography is decided after 6 months (Fig. 4).
Recommendations for management of MUA
The American Heart Association Stroke Council documented certain recommendations for factors favoring the treatment of UA after reviewing the literature. The young age patients and those with previous history of SAH or having family members with aneurysmal SAH should be treated. The aneurysmal factors that favor treatment are as follows: basilar apex location, size more than or equal 10 mm, and presence of daughter sacs. For patients for whom conservative management is elected, routine periodic angiography should be done. Patient with documented aneurysmal growth radiological or clinical (development of new symptoms) should be considered for intervention as mentioned before [54].
In case of MUA, all aneurysms should be treated when technically viable. In case of selection of certain aneurysms to start with, our recommendation is summarized in a flowchart (see Fig. 5). Selection is based on aneurysmal radiological signs in correlation with the clinical presentation. Tailoring the protocol therapy by combining variable treatment options available and creating a multimodal approach with the integration of surgeons and interventionists expertise is inevitable.
Conclusion
Prophylactic treatment of multiple unruptured is still controversial. Weighing the risk of intervention to the risk of observation is a mandatory pathway. However, the favorable outcome of treating these aneurysms in comparison with the ruptured one and sparing the patients the drawbacks of impending rupture and subsequent SAH favors intervention. Multiplicity and other factors like age of patients, size and location of the aneurysms influence the decision-making and the type of therapy to be elected.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Change history
08 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s41984-023-00206-z
Abbreviations
- ACOM:
-
Anterior communicating artery
- CA:
-
Cerebral aneurysms
- CTA:
-
CT angiography
- ISUIA:
-
International Study of Unruptured Intracranial Aneurysms
- MCA:
-
Middle cerebral artery
- MRA:
-
Magnetic resonance angiography
- MUA:
-
Multiple unruptured aneurysm
- PCOM:
-
Posterior communicating artery
- SAH:
-
Subarachnoid hemorrhage
- TIA:
-
Transient ischemic attack
- UA:
-
Unruptured aneurysm
References
Jakubowski J, Kendall BR. Coincidental aneurysms with tumours of pituitary origin. J Neurol Neurosurg Psychiatry. 1978;41(11):972–9.
Connolly ES Jr, Solomon RA. Management of symptomatic and asymptomatic unruptured aneurysms. Neurosurg Clin N Am. 1998;9(3):509–24.
Schievink WI. Genetics of intracranial aneurysms. Neurosurgery. 1997;40(4):651–63.
Broderick JP, Sauerbeck LR, Foroud T, Huston J, Pankratz N, Meissner I, Brown RD. The familial intracranial aneurysm (FIA) study protocol. BMC Med Genet. 2005;6(1):1–9.
Jabbarli R, Dinger TF, Darkwah Oppong M, Pierscianek D, Dammann P, Wrede KH, Kaier K, Köhrmann M, Forsting M, Kleinschnitz C, Sure U. Risk factors for and clinical consequences of multiple intracranial aneurysms: a systematic review and meta-analysis. Stroke. 2018;49(4):848–55.
Jl C, Wm H. Berry aneurysms of the circle of Willis; results of a planned autopsy study. Neurology. 1958;8(1):41–4.
Wiebers DO, International Study of Unruptured Intracranial Aneurysms Investigators. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.
Williams LN, Brown RD. Management of unruptured intracranial aneurysms. Neurol Clin Pract. 2013;3(2):99–108.
Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med. 2006;355(9):928–39.
Greenberg MS. Unruptured aneurysms. In: Handbook of neurosurgery. New York: Thieme Medical Publishers; 2010. p. 1077–9.
Morris P. Practical neuroangiography. Philadelphia: Lippincott Williams & Wilkins; 2007.
Tampieri D, Leblanc R, Oleszek J, Pokrupa R, Melançon D. Three-dimensional computed tomographic angiography of cerebral aneurysms. Neurosurgery. 1995;36(4):749–55.
Villablanca JP, Duckwiler GR, Jahan R, Tateshima S, Martin NA, Frazee J, Gonzalez NR, Sayre J, Vinuela FV. Natural history of asymptomatic unruptured cerebral aneurysms evaluated at CT angiography: growth and rupture incidence and correlation with epidemiologic risk factors. Radiology. 2013;269(1):258–65.
Gibbs GF, Huston J III, Bernstein MA, Riederer SJ, Brown RD Jr. 3.0-Tesla MR angiography of intracranial aneurysms: comparison of time-of-flight and contrast-enhanced techniques. J Magn Reson Imaging. 2005;21(2):97–102.
Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery. 2003;52(3):624–31.
Takao H, Nojo T, Ohtomo K. Screening for familial intracranial aneurysms: decision and cost-effectiveness analysis. Acad Radiol. 2008;15(4):462–71.
Washington CW, Vellimana AK, Zipfel GJ, Dacey RG. The current surgical management of intracranial aneurysms. J Neurosurg Sci. 2011;55(3):211–31.
Polevaya NV, Kalani MY, Steinberg GK, Victor CK. The transition from hunterian ligation to intracranial aneurysm clips: a historical perspective. Neurosurg Focus. 2006;20(6):1–7.
Britz GW. Clipping or coiling of cerebral aneurysms. Neurosurg Clin. 2005;16(3):475–85.
Sorenson T, Lanzino G. Newer endovascular tools for treatment of intracranial aneurysms: a primer for neurosurgeons. Contemp Neurosurg. 2015;37(22):1–5.
Weber W, Siekmann R, Kis B, Kuehne D. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. Am J Neuroradiol. 2005;26(8):1909–15.
Seibert B, Tummala R, Chow R, Faridar A, Mousavi SA, Divani AA. Intracranial aneurysms: review of current treatment options and outcomes. Front Neurol. 2011;8(2):45.
Sandström N, Yan B, Dowling R, Laidlaw J, Mitchell P. Comparison of microsurgery and endovascular treatment on clinical outcome following poor-grade subarachnoid hemorrhage. J Clin Neurosci. 2013;20(9):1213–8.
Ajiboye N, Chalouhi N, Starke RM, Zanaty M, Bell R. Unruptured cerebral aneurysms: evaluation and management. Sci World J. 2015;2015:1.
Vajda J. Multiple intracranial aneurysms: a high risk condition. Acta Neurochir. 1992;118(1):59–75.
Wilson FM, Jaspan T, Holland IM. Multiple cerebral aneurysms: a reappraisal. Neuroradiology. 1989;31(3):232–6.
Heiskanen O, Marttila I. Risk of rupture of a second aneurysm in patients with multiple aneurysms. J Neurosurg. 1970;32(3):295–9.
King JT, Berlin JA, Flamm ES. Morbidity and mortality from elective surgery for asymptomatic, unruptured, intracranial aneurysms: a meta-analysis. J Neurosurg. 1994;81(6):837–42.
Morita A, Teramoto A. The natural course of unruptured cerebral aneurysms: natural course analysis of the unruptured cerebral aneurysm study in Japan. J Neurosurg. 2012;117(2):A386–A386.
Wermer MJ, van der Schaaf IC, Algra A, Rinkel GJ. Risk of rupture of unruptured intracranial aneurysms in relation to patient and aneurysm characteristics: an updated meta-analysis. Stroke. 2007;38(4):1404–10.
Dell S. Asymptomatic cerebral aneurysm: assessment of its risk of rupture. Neurosurgery. 1982;10(2):162–6.
Juvela S, Poussa K, Lehto H, Porras M. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. Stroke. 2013;44(9):2414–21.
Orz Y, Kobayashi S, Osawa M, Tanaka Y. Aneurysm size: a prognostic factor for rupture. Br J Neurosurg. 1997;11(2):144–9.
Juvela S, Porras M, Heiskanen O. Natural history of unruptured intracranial aneurysms: a long-term follow-up study. J Neurosurg. 1993;79(2):174–82.
Yasui N, Suzuki A, Nishimura H, Suzuki K, Abe T. Long-term follow-up study of unruptured intracranial aneurysms. Neurosurgery. 1997;40(6):1155–60.
Mizoi K, Yoshimoto T, Nagamine Y, Kayama T, Koshu K. How to treat incidental cerebral aneurysms: a review of 139 consecutive cases. Surg Neurol. 1995;44(2):114–20.
Broderick JP, Brown RD Jr, Sauerbeck L, Hornung R, Huston J III, Woo D, Anderson C, Rouleau G, Kleindorfer D, Flaherty ML, Meissner I. Greater rupture risk for familial as compared to sporadic unruptured intracranial aneurysms. Stroke. 2009;40(6):1952–7.
Wiebers DO, Whisnant JP, Sundt TM, O’Fallon WM. The significance of unruptured intracranial saccular aneurysms. J Neurosurg. 1987;66(1):23–9.
Orz YI, Hongo K, Tanaka Y, Nagashima H, Osawa M, Kyoshima K, Kobayashi S. Risks of surgery for patients with unruptured intracranial aneurysms. Surg Neurol. 2000;53(1):21–9.
Chen PR, Frerichs K, Spetzler R. Current treatment options for unruptured intracranial aneurysms. Neurosurg Focus. 2004;17(5):1–6.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, Sandercock P. International subarachnoid aneurysm trial (ISAT) Collaborative Group. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17.
Feng Z, Fang Y, Xu Y, Hong B, Zhao W, Liu J, Huang Q. The safety and efficacy of low profile visualized intraluminal support (LVIS) stents in assisting coil embolization of intracranial saccular aneurysms: a single center experience. J NeuroIntervent Surg. 2016;8(11):1192–6.
Shen X, Xu T, Ding X, Wang W, Liu Z, Qin H. Multiple intracranial aneurysms: endovascular treatment and complications. Interv Neuroradiol. 2014;20(4):442–7.
Oishi T, Takashima K, Yoshinaka K, Yu K, Ohta M, Mori K, Toma N. Evaluation of effect of aneurysm model material on coil contact force and catheter movement. J Biomech Sci Eng. 2022;17(1):21–00261.
Molyneux AJ, Cekirge S, Saatci I, Gál G. Cerebral aneurysm multicenter european onyx (CAMEO) trial: results of a prospective observational study in 20 European centers. Am J Neuroradiol. 2004;25(1):39–51.
Kallmes DF, Hanel R, Lopes D, Boccardi E, Bonafé A, Cekirge S, Fiorella D, Jabbour P, Levy E, McDougall C, Siddiqui A. International retrospective study of the pipeline embolization device: a multicenter aneurysm treatment study. Am J Neuroradiol. 2015;36(1):108–15.
Brouillard AM, Sun X, Siddiqui AH, Lin N. The use of flow diversion for the treatment of intracranial aneurysms: expansion of indications. Cureus. 2016. https://doi.org/10.7759/cureus.472.
Dmytriw AA, Salem MM, Yang VX, Krings T, Pereira VM, Moore JM, Thomas AJ. Endosaccular flow disruption: a new frontier in endovascular aneurysm management. Neurosurgery. 2020;86(2):170–81.
Choudhri O, Mukerji N, Steinberg MD, Gary K. Combined endovascular and microsurgical management of complex cerebral aneurysms. Front Neurol. 2013;8(4):108.
Juvela S. Alcohol and the prognosis of subarachnoid hemorrhage. Duodecim; laaketieteellinen aikakauskirja. 1993;109(5):355–7.
Tarlov N, Norbash AM, Nguyen TN. The safety of anticoagulation in patients with intracranial aneurysms. J NeuroIntervent Surg. 2013;5(5):405–9.
Toussaint LG, Friedman JA, Wijdicks EF, Piepgras DG, Pichelmann MA, McIver JI, McClelland RL, Nichols DA, Meyer FB, Atkinson JL. Influence of aspirin on outcome following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101(6):921–5.
Thompson BG, Brown RD Jr, Amin-Hanjani S, Broderick JP, Cockroft KM, Connolly ES Jr, Duckwiler GR, Harris CC, Howard VJ, Johnston SC, Meyers PM. Guidelines for the management of patients with unruptured intracranial aneurysms: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46(8):2368–400.
Orz YI. Unruptured anterior communicating artery aneurysm. In: Sabbagh AJ, editor. Neurosurgery case review: questions and answers. Thieme; 2009. p. 109
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MD designed the study and reviewed the literature and revised the final draft. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: the author reported an error in the Tables 1, 2 caption cited references.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deniwar, M.A. Management of multiple and unruptured cerebral aneurysms. Egypt J Neurosurg 37, 26 (2022). https://doi.org/10.1186/s41984-022-00170-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41984-022-00170-0




